Freeze Drying Theory
Freeze drying or lyophilization, is a low temperature dehydration process. Dry products in a solid state and is under vacuum environment. Typically use to preserve perishable materials or make the material more convenient for storage.
Definition of Freeze Drying
Freeze drying, is a dehydration process typically use to preserve perishable materials or make the material more convenient for storage. In pharmaceutical area, freeze drying called lyophilization, they are synonymous.
The definition of freeze drying or lyophilization is to dry something in a solid state and is under vacuum environment.
Thus, freeze drying removes water by freezing the material, and then reduce its surrounding pressure, proper apply heat to allow the frozen water in the material to sublimate directly from solid phase to gas.
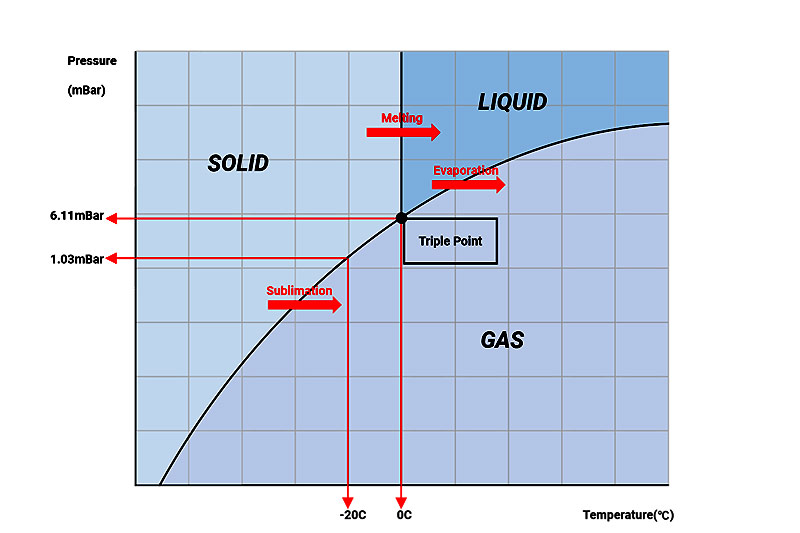
The Water 3 Phases
Normally, there are 3 forms of water (vapor, solid, liquid) in environment, and any 2 of the 3 can flexible convert, by apply pressure and temperature to. Vapor convert to solid called condensing, solid convert to vapor called sublimation, solid convert to liquid called melting.
For freeze drying, it mainly involves intersections of vapor and solid. That is: When solid water (Temp.<0C) surrounding pressure is lower than triple point (6.11mbar), it will pass through liquid phase, sublimate to gas directly.
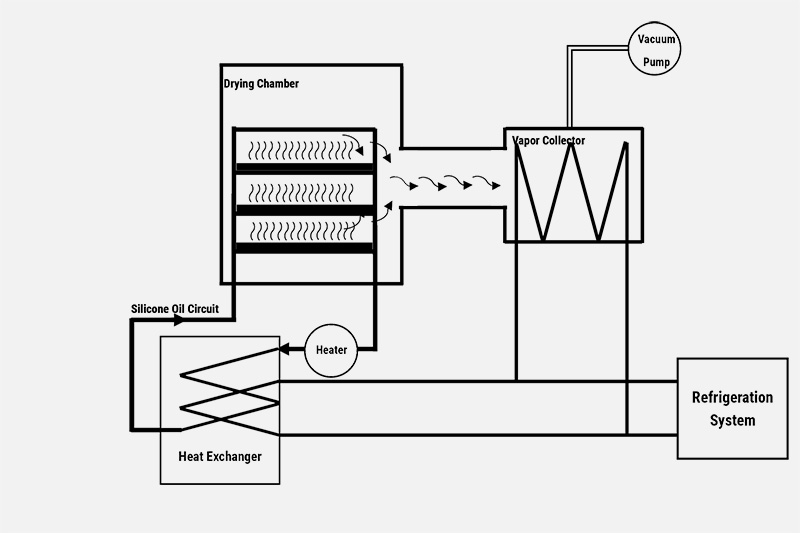
How a Freeze Dryer Process Freeze Drying
Base on the principle of freeze drying and the conversion requirement of water phases, freeze dry systems are built capable of change water temperature and its surrounding pressure. Create sublimation conditions, to enable water removes from raw product.
In a freeze drying equipment,
- Drying chamber is the place where raw product are placed, chilled and dried.
- Ice condenser is use to collect the sublimated vapors.
- Vacuum pumps are use to vacuumize and maitain the drying chamber pressure.
- Compressors are use to supply constant cooling energy to condenser, guarantee it can continually condensing vapors.
- Control system ensures the automatic freeze drying process.